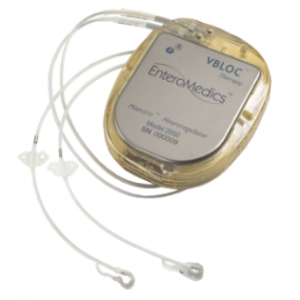
 The Food and Drug Administration (FDA) approved a new device for obesity called VBLOC.
The Food and Drug Administration (FDA) approved a new device for obesity called VBLOC.
The device produces an electrical stimulation to the vagus nerve, with the theory that this signals the brain that the stomach is full.
Early Results
Some patients had the device implanted, but did not have the electrodes turned on. Those patients lost 16% of their excess weight, while the patients who had the electrodes turned on lost 24% of their excess weight. The target that the company had set was the patients who had the electrodes turned on would lose at least ten per cent more than those who did not. This means, some patients lost a lot of excess weight and other did not.
But rest assured- without lifestyle changes there is no weight loss operation that will work.
So while the device works well, clearly the majority of the weight loss (66%) came from other factors – no doubt the lifestyle changes that were incorporated with the device. Here is the trick- without lifestyle changes there is no weight loss surgery that will work. Lifestyle changes are the basis of weight loss surgery. Surgery is successful in patients when they change what they eat, how they eat, and especially when they get into the kitchen and cook.
Without changing lifestyle- no weight loss surgery works by itself. Some weight loss operations allow patients to lose weight quickly, without changing – but as time goes by if they do not change, they will regain weight. Of all weight loss operations out there- all of them fail for one of two reasons:
The stomach stretches (the RNY anastomosis opens up so those patients don’t have satiety).
The stomach stretches (so the sleeve no longer either restrictive and the hormone secreting ghrelin cells have hypertrophied)
The stomach stretches (above the Lap-Band and patients begin to get slips, erosions and lose their ‘satiety’)
There is no stomach to stretch – it is pure nerve interruption.
To translate what the study showed: if you had 100 pounds to lose, and followed the program outlined for these patients, and had a device implanted that didn’t work, you would lose an average of 16 pounds. If you have the device implanted you would lose an average of 24 pounds (8.5% more). The placebo effect of having a device, believing it is doing something for you, and generally following a better lifestyle led to a weight loss that was the majority of the weight from surgery. But it isn’t placebo effect- it is lifestyle change. And that is the “average” of a population of weight loss patients – which means some lost less and many lost more.
The device requires an operation to implant electrodes on the stomach, as well as a battery pack- similar to a pacemaker. This battery must be changed periodically and is contraindicated in people who might anticipate needing a MRI, lithotripsy for stones.
When the FDA voted to determine if it was effective, the vote was no (vote by the advisory panel 5 no and 4 yes)- however, since the device had some efficacy, and was considered safe (by a 8-1 vote) the device was ultimately approved.
Would I recommend this device for patients who are obese? Yes.
Now let me qualify this. I would recommend this device to some obese patients, because when you say a 24% excess weight loss average that means there are those who do much better. The trick will be to define who those people might be good candidates for this. Or helping those who get the device, learn to use it. When we see patients who are obese, there are those who say the main issue is hunger – tremendous hunger, and if we could curb that they would eat less. The operation is considered safe, that small amount of weight loss does not seem worth an operation and the limitations that this would place on an individual (see the issues below) – however, if we could define those who might benefit from this- the people who say they are hungry – well, this would be a win for them.
Getting rid of hunger means being able to turn down unhealthy foods – and being satisfied with less. It is the holy grail of weight loss. For some patients, with appropriate commitment to lifestyle changes – this is a device that will work well. Who those patients are? I suspect those who say they are “always hungry” or “never full.” As opposed to those who eat poorly constantly.
Moving Forward Lets Find How To Determine Who This Works Best With
The FDA wants to see longer term results, and the company has agreed to this. The most interesting part would be to see a food profile of those who do well with this device? Are they sweet eaters, volume eaters (my guess), do they like to cook. What are the profiles of those who do well with the device.
We may never know- but I suspect with some good research we will find out.
Below is from the company’s website:
Seek guidance from your doctor before you undergo a medical or surgical procedure, as interaction of the Maestro System with certain medical therapies, procedures or other implanted or body worn medical devices may harm you, cause damage to the implanted device or may turn therapy off. These may include, but are not limited to, lithotripsy (use of high energy shock waves to break up stones), radiation, mono polar electrosurgical instruments (a type of surgical instrument), positron emission tomography scans (a type of medical imaging), radiofrequency ablation (a method of destroying tissue used during surgery), heart pacemakers or defibrillators, neurostimulators, and insulin pumps. Turning, twisting, or manipulating the implanted components may damage the nerves or implanted device. System components must be kept charged to prevent damage, which may require additional surgery to replace the implanted device. The neuroregulator should be fully charged prior to turning it off. The Maestro System is MR Unsafe, including for patients in which the Maestro System was explanted and not all components were removed. Portable outlets or extension cords should not be connected to the AC recharger. Do not immerse external system components in fluid. Keep strong magnets at least 6 inches away from the implanted device. The Maestro Rechargeable System may activate metal detectors or other security systems. Strong magnetic fields systems that emit radio frequency signals may interfere with the function of the Maestro System. The neuroregulator and mobile charger should be turned off near metal detectors, other security systems, strong magnetic fields and radio frequency emitting systems. The mobile charger should be turned off while aboard aircraft. Infection at the implant site may occur and could require use of antibiotic medications, surgery, or explant. Do not modify any components of the system. Safety and effectiveness of the Maestro Rechargeable System has not been established for use within a hyperbaric chamber (chamber designed to supply oxygen at a higher than normal air pressure), with external defibrillation, during pregnancy, or for use in patients under 18 years of age. The capacity of the rechargeable neuroregulator battery will diminish over time, requiring longer or more frequent charging. Do not operate the system in flammable environments, or if components appear damaged. Do not cover the mobile charger when in use to prevent overheating and damage. You may not be able to operate the Maestro System if you have impaired vision. Make sure all of your health care providers are aware that you have an implanted Maestro System.